New Regulations
The Americans with Disabilities Act of 1990 (ADA) and Section 504 of the Rehabilitation Act of 1973 are federal civil rights laws that prohibit discrimination against individuals with disabilities in every-day activities, including equal access to medical services.
While the ADA was passed in 1990 no real progress in establishing concrete specifications for medical devices intended to remove barriers to healthcare for those with mobility disabilities was established until the Affordable Care Act of 2010 (Obamacare).
The ACA mandated that the federal entity responsible for promulgating such regulations, The United States Access Board, write those standards within a certain period of time.
The U.S. Access Board stated its’ medical device rule making activities in 2012, and in January 2017, the Access Board added part 1195 to title 36 of the Code of Federal Regulations as a published document in the Federal Register.
Currently, these regulations are voluntary until they are adopted as mandatory by an enforcing authority such as the Department of Justice. In the absence of the Access Board regulations, the Department of Justice actually established its’ own, almost identical standards in July of 2010, which they can and have enforced through the federal courts.
The United States Department of Veterans Affairs has already adopted the new Access Board regulations as their standard for medical equipment procurement. Several states have also indicated they intend to adopt these new standards to regulate access to healthcare by patients with mobility disabilities.
The following is a partial summary of those regulations as they relate to MTI’s equipment. Click the links below for the complete text of the regulations and the illustrations, which have been provided by the United States Access Board.
Links:
36 CFR Part 1195
RIN 3014–AA40
Standards for Accessible Medical Diagnostic Equipment
Summary
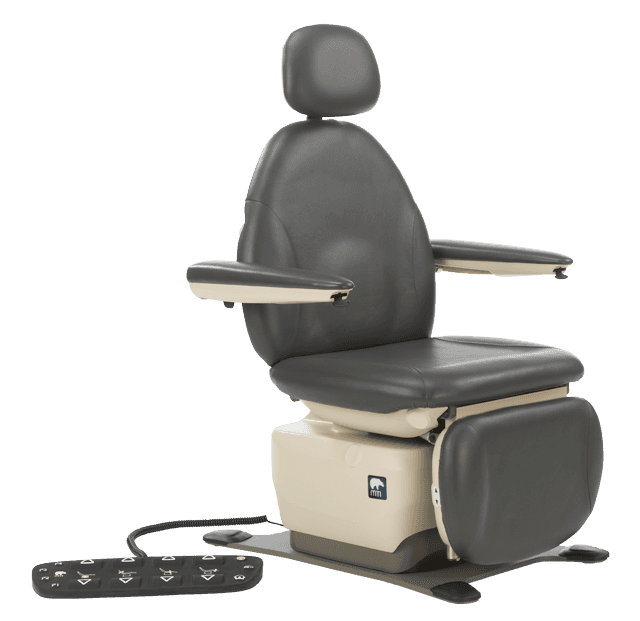
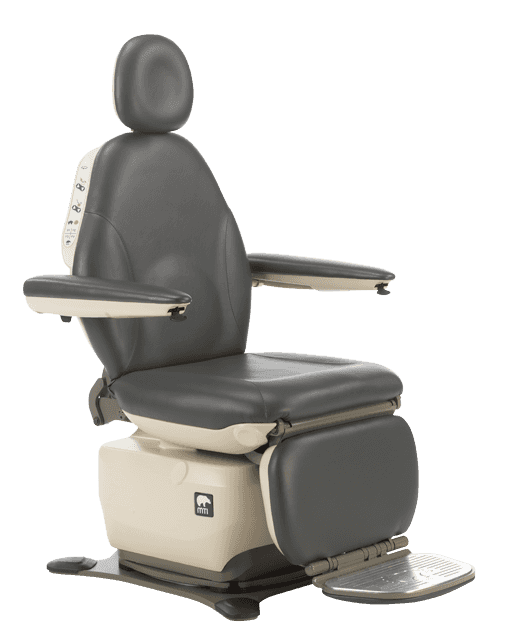
The Access Board has divided the Standards for Accessible Medical Diagnostic Equipment (MDE) into separate technical criteria based on how the equipment is used by the patient. Medical equipment for supine, prone, or side laying positions, such as those for general examination, GYN and OB, are categorized as M301 equipment. Medical equipment intended for positions initially in the seated position such as ENT, Podiatry, Dermatology, Plastic Surgery, Ophthalmology, Oral Surgery and others, are categorized as M302 equipment. Medical equipment which can be accessed while the patient remains in their wheeled mobility device, such as mammography equipment and weight scales, are categorized as M303 equipment.
- 17-19” entry height: A low transfer adjustability height of 17”-19” and a high transfer height of at least 25” for M301 and M302 equipment. The method of measure for the 17”-19” measurement is measured from the floor to the highest point, including bolsters, of the transfer surface with the upholstery in an “uncompressed” (relaxed) condition.
- 21×17” transfer surface: M302 equipment must have a transfer surface at least 21” wide and 17” deep.
- 2 transfer sides: M302 medical chairs must provide transfer on two adjoining sides (front and side) of the transfer surface. There is an exception for M302 equipment which permits transfer from a mobility device onto opposing sides of the transfer surface where the transfer surface is obstructed by a fixed footrest, such as ENT, Dental and Podiatry chairs.
- 15” transfer support: A “Transfer Support” must meet the following specifications in M305.1-5: minimum of 15” length, maximum transfer support position of 1.5” from
the horizontal transfer surface, and covering a minimum 13.5” depth of the transfer surface.
MTI’s 829, 830, 840, 550 & 464 procedure and exam chairs…
meet all of the applicable regulations for 36 CFR Part 1195 Standards for Accessible Medical Diagnostic Equipment for standard, swivel and mobile bases.